Understanding Car Soap Ingredients and Their Impact on PPF Maintenance
Proper care for your vehicle's paint protection film (PPF) relies on selecting the right car soaps, especially those labeled as pH-neutral and free from harsh detergents. To make informed choices, it’s essential to understand the roles of surfactants and detergents in automotive soaps and their potential effects on PPF.
What Are Surfactants and Detergents?
Surfactants
- Definition: Surfactants (short for surface-active agents) are chemical compounds that reduce the surface tension of water, allowing it to spread and penetrate surfaces more effectively.
- How They Work:
- Surfactants have two parts: a hydrophilic (water-attracting) head and a hydrophobic (oil-attracting) tail.
- This dual nature allows surfactants to bind with water on one end and oils, grease, or dirt on the other, helping lift and wash away contaminants.
- In Automotive Soaps: Surfactants play a key role in cleaning by loosening dirt, grime, and oils from the vehicle's surface. They also contribute to foam and lather, which improve the soap's application and rinsing performance.
Detergents
- Definition: Detergents are a subclass of surfactants designed specifically to clean surfaces by breaking down and removing oils, grease, and dirt.
- Relation to Surfactants: While all detergents are surfactants, not all surfactants are detergents. Detergents are formulated with additional cleaning agents to enhance their effectiveness.
- In Automotive Soaps: Detergents are responsible for deep cleaning but can sometimes strip protective layers like wax, sealants, or even degrade PPF if overly aggressive.
How Surfactants and Detergents Relate to Automotive Soaps
- Foaming Action: Surfactants create foam, which helps lift contaminants from the surface.
- Grease Cutting: Detergents enhance the soap’s ability to break down stubborn oils and grease.
- Potential Harshness: Strong detergents or improperly formulated surfactants can remove protective coatings, such as wax or sealants, and may degrade the integrity of PPF over time.
- Mild Alternatives: Gentle surfactants and detergents, like Cocamidopropyl Betaine or Cocamidopropyl Hydroxysultaine, are designed to clean without compromising the PPF or protective layers.
Ingredient Analysis in Car Soaps
Water (7732-18-5)
- Role: A solvent that forms the base of the formula, dissolving and carrying active ingredients.
- Effect: Harmless and non-harsh.
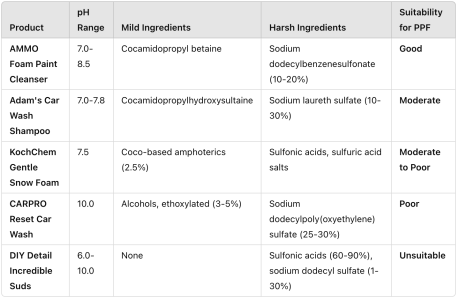
Sodium Dodecylbenzenesulfonate (25155-30-0)
- Type: Detergent surfactant.
- Function: Provides grease-cutting and foaming properties.
- Concerns:
- Can strip wax, sealants, and degrade PPF with frequent use or high concentrations.
- Harsh on delicate surfaces.
- Verdict: Potentially harsh; use only in PPF-specific formulations.
Sodium Alpha Olefin Sulfonate (68439-57-6)
- Type: Milder surfactant.
- Function: Effective for cleaning and foaming.
- Concerns:
- Less harsh than some detergents but may still damage PPF with overuse.
- Verdict: Moderately harsh depending on concentration.
Cocamidopropyl Betaine
- Type: Mild surfactant derived from coconut oil.
- Function: Balances harsh detergents, reducing irritation and harsh effects.
- Effect: Safe and suitable for PPF.
- Verdict: Mild and PPF-friendly.
Sodium Laureth Sulfate (SLES)
- C.A.S. Numbers: 68585-34-2 / 68891-38-3
- Type: Detergent surfactant.
- Function: Provides strong cleaning and foaming properties.
- Concerns:
- Aggressive grease-cutting action can strip protective coatings or damage PPF over time.
- Verdict: Harsh if not properly balanced or diluted in a PPF-safe formulation.
Cocamidopropyl Hydroxysultaine (CHS)
- C.A.S. Number: 68139-30-0
- Type: Mild amphoteric surfactant.
- Function: Enhances the gentleness of a formula, providing effective cleaning while protecting surfaces.
- Effect: Suitable for PPF, especially in pH-neutral soaps.
- Verdict: Gentle and highly recommended for PPF maintenance.
Choosing the Right Car Soap for PPF
- Prioritize pH-Neutral Formulations: Soaps with balanced pH levels are less likely to harm PPF or protective coatings.
- Avoid Harsh Detergents: Ingredients like Sodium Dodecylbenzenesulfonate and SLES can be aggressive. Look for milder alternatives.
- Check for PPF-Safe Labels: Ensure the product explicitly states compatibility with PPF.
Chemical Identification and Safety
C.A.S. Number (Chemical Abstracts Service)
- Purpose: Provides a unique identifier for chemical substances, ensuring clarity in research and safety data.
- Format: A numeric identifier (e.g., 7732-18-5 for water).
- Importance: Helps in identifying ingredients in product safety sheets.
PEL (Permissible Exposure Limit)
- Definition: OSHA's regulatory limit on airborne exposure to chemicals over an 8-hour workday.
- Application: Ensures safe exposure levels in workplaces.
In Summary
Understanding the roles of surfactants and detergents in car soaps is key to protecting your vehicle’s PPF. While strong detergents like SLES can aggressively clean, they may damage protective layers if not properly formulated. Opt for pH-neutral soaps containing mild surfactants like Cocamidopropyl Betaine or CHS to maintain your PPF's integrity and appearance. Always verify product labels for PPF compatibility to ensure long-lasting protection.What other soaps should I include in the poll?
Last edited: